- Show results for
- Share
What is Viscosity and How to Measure It?
Resource Description

What is Viscosity and Its Formula
Viscosity is a significant criterion that allows for measuring the flow of fluids such as gasses, solids, and liquids. Viscosity relates to fluid thickness. The Viscosity results are obtained from the contact or difference between the molecules in a fluid. It is similar to the difference between moving solids when the viscosity will identify the energy that is needed to provide the flow of fluids.
There are two types of viscosity: dynamic and kinematic.
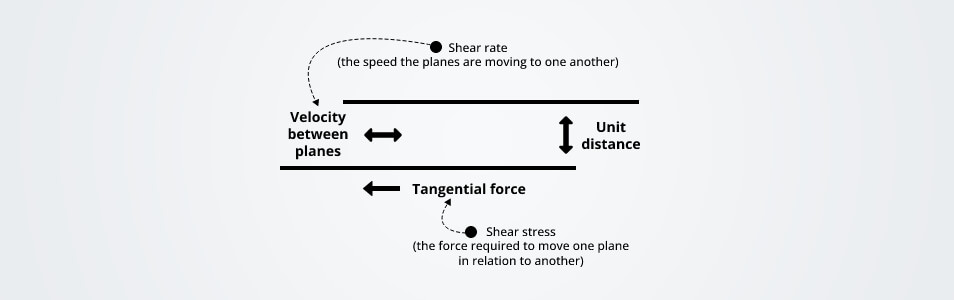
Dynamic viscosity is a strength that is essential for fluid to overtake its inner molecular friction thus making the fluid flow. Simply, dynamic viscosity is specified as the tangential strength per unit area that is needed to move the fluid in one horizontal plane with support to the other plane with a velocity unit while the fluid’s molecules maintain a unit distance apart
Dynamic viscosity is also specified as the absolute viscosity in the fluid mechanic's sphere.
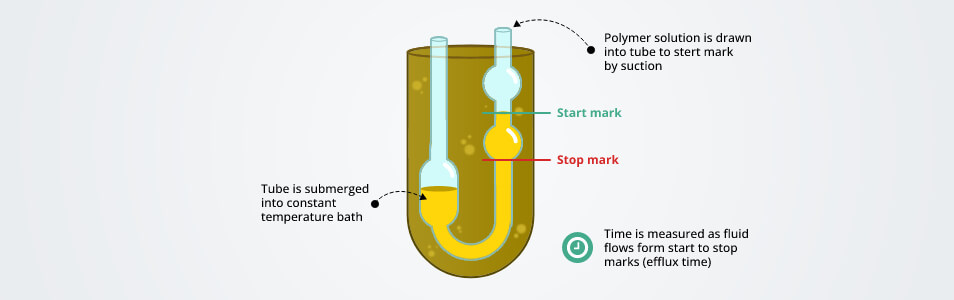
Kinematic viscosity helps to measure fluid’s inner resistance that flows under the gravitational strengths. It is observed by measuring the time in seconds needed for an attached volume of fluid to flow a known distance by gravity through a capillary within a calibrated device at a closely controlled temperature.
In physics, viscosity is often indicated as Isaac Newton’s comparison of fluids, which is comparable to Newton’s second law of motion. This law explains the action of the strength on an object, thus causing the object to speed up. The bigger the weight of the object, the more increased the strength will be essential to cause it to speed up.
Viscosity Formula
The viscosity formula is often explained by Isaac Newton’s comparison of fluids:
| F / A = n (dv / dr)* |
where F is strength and A is area. When strength is divided by area is the other way of determining viscosity. DV divided by dr shows the “shear rate”, or the moving speed of the liquid. The n is a constant gauging unit that is equal to 0.00089 Pa s, or as it is also explained as Pascal-second, which is a gauging unit for the dynamic viscosity. This law is helpful for different practical applications such as protein formulations or injections, and food and beverage manufacturings.
Nowadays, a lot of manufacturers consider viscometers as a significant part of their observations, developments, and process control programs. They understand that viscosity gauging is often the most precise, durable, and quickest way to determine some of the most crucial aspects that impact a product’s performance.
How Is Viscosity Measured?
The viscosity of the solution is measured with a viscometer. The best viscometers are those that may be able to generate and control simple flow fields.
The most common standard viscometers are constructed in a U-shape. Such a device is usually made of glass. The wider limb of the viscometer is placed on the side of the viscometer’s capillary tube and functions as the opening for the fluid that is needed to be measured is poured.
The viscometer generally included two bulbs that are connected with a support of a U-shaped tube. One of these bulbs is attached to the capillary tube on one side and the rubber tube on the other side. The other bulb includes two etched markings on either side. These markings are maintained to monitor the limit of the flow of the liquids or solutions that pass through the capillary tube. In addition, these two markings are typically identified by upper Mark-A and lower mark-B. The other opening of the viscometer tube is connected to a rubber tube and is used to press the liquid inside.
There are a lot of different ways to measure viscosity. Some of the most common ways are:
The capillary viscometer is one of the oldest ways to measure viscosity. The capillary viscometer measures the time between the volume of the liquid that passes through the distance of the capillary tubes.
A rotational viscometer measures the torque that is needed to rotate the object within the volume of the liquid.
Falling Sphere Viscometer helps to measure the viscosity by decreasing the sphere of the specific mass and density and to measure the time that the sphere takes to achieve designated junctures.
Zahn Cup Method allows measuring the viscosity by determining the time that the volume of the liquid takes to empty the cup that is placed in the bottom of the viscometer’s container.
A vibrational viscometer measures the vibrational waves with the use of a vibrating rod that is submerged in the fluid. After that, the viscosity is measured by calculating the vibration dampening.
Viscosity Chart
Counting the viscosity of the fluids you need to measure may be puzzling and quite complicated. That is why we offer you a high-quality Viscosity Reference Chart that may help you to calculate the viscosity of the liquid effortlessly.
In layman’s terms, the viscosity determines the fluid’s resistance flow. Simply, the higher the viscosity of the liquid or solution is, the thicker and the more increased the resistance flow. The temperature will influence the viscosity of most liquids and solutions.
| Fluid | Specific Gravity | Viscosity CPS | Viscous Type | |
| Reference | Water | 1.0 | 1.0 | Newtonian |
|
Adhesives |
"Box" Adhesives | 1+- | 3,000 | Thixotropic |
| PVA | 1.3 | 100 | Thixotropic | |
| Rubber & Solvents | 1.0 | 15,000 | Newtonian | |
|
Bakery |
Batter | 1.0 | 2.000 | Thixotropic |
| Butter (Melted) | 0.98 | 18 @ 140 °F | Newtonian | |
| Egg (Whole) | 0.5 | 60 @ 50 °F | Newtonian | |
| Emulsifier | - | 20 | Thixotropic | |
| Frosting | 1.0 | 10,000 | Thixotropic | |
| Lectithin | - | 3,250 @ 125 °F | Thixotropic | |
| 77% Sweetened Condensed Milk | 1.3 | 10,000 @ 77°F | Newtonian | |
| Yeast Slurry 15% | 1.0 | 180 | Thixotropic | |
|
Beer |
Beer | 1.0 | 1.1 @ 40°F | Newtonian |
| Brewers Concentrated Yeast (80% solids) | - | 16,000 @ 40°F | Thixotropic | |
|
Confectionery |
Caramel | 1.2 | 400 @ 140°F | Thixotropic |
| Chocolate | 1.1 | 17,000 @ 120°F | Thixotropic | |
| Fudge (Hot) | 1.1 | 36,000 | Thixotropic | |
| Toffee | 1.2 | 87,000 | Thixotropic | |
|
Cosmetics/Soaps |
Face Cream | - | 10,000 | Thixotropic |
| Hair Gel | 1.4 | 5,000 | Thixotropic | |
| Shampoo | - | 5,000 | Thixotropic | |
| Toothpaste | - | 20,000 | Thixotropic | |
| Hand Cleaner | - | 2,000 | Thixotropic | |
|
Dairy |
Cottage Cheese | 1.08 | 225 | Thixotropic |
| Cream | 1.02 | 20 @ 40°F | Newtonian | |
| Milk | 1.03 | 1.2 @ 60°F | Newtonian | |
| Process Cheese | - | 30,000 @ 160°F | Thixotropic | |
| Yogurt | - | 1,100 | Thixotropic | |
| Detergents | Detergent Concentrate | - | 10 | Newtonian |
|
Dyes & Inks |
Printers Ink | 1 to 1.38 | 10,000 | Thixotropic |
| Dye | 1.1 | 10 | Newtonian | |
| Gum | - | 5,000 | Thixotropic | |
|
Fats & Oils |
Corn Oil | 0.92 | 30 | Newtonian |
| Lard | 0.96 | 60 @ 100°F | Newtonian | |
| Linseed Oil | 0.93 | 30 @ 100°F | Newtonian | |
| Peanut Oil | 0.92 | 42 @ 100°F | Newtonian | |
| Soybean Oil | 0.95 | 36 @ 100°F | Newtonian | |
| Vegetable Oil | 0.92 | 3 @ 300°F | Newtonian | |
|
Misc. Foods |
Black Bean Paste | - | 10,000 | Thixotropic |
| Cream Style Corn | - | 130 @ 190°F | Thixotropic | |
| Catsup (Ketsup) | 1.11 | 560 @ 145°F | Thixotropic | |
| Pablum | - | 4,500 | Thixotropic | |
| Pear Pulp | - | 4,000 @ 160°F | Thixotropic | |
| Mashed Potato | 1 | 20,000 | Thixotropic | |
| Potato Skins & Caustic | - | 20,000 @ 100°F | Thixotropic | |
| Prune Juice | 1 | 60 @ 120°F | Thixotropic | |
| Orange Juice Concentrate | 1.1 | 5,000 @ 38°F | Thixotropic | |
| Tapioca Pudding | 0.7 | 1,000 @ 235°F | Thixotropic | |
| Mayonnaise | 1 | 5,000 @ 75°F | Thixotropic | |
| 33% Tomato Paste | 1.14 | 7,000 | Thixotropic | |
| Honey | 1.5 | 1,500 @ 100°F | Thixotropic | |
|
Meat Products |
Melted Animal Fats | 0.9 | 43 @ 100°F | Newtonian |
| Ground Beef Fats | 0.9 | 11,000 @ 60°F | Thixotropic | |
| Meat Emulsion | 1 | 22,000 @ 40°F | Thixotropic | |
| Pet Food | 1 | 11,000 @ 40°F | Thixotropic | |
| Pork Fat Slurry | 1 | 650 @ 40°F | Thixotropic | |
| Misc. Chemicals | Glycols | 1.1 | 35 @ Range | - |
|
Paint |
Metallic Auto Paints | - | 220 | Thixotropic |
| Solvents | 0.8 to 0.9 | 0.5 to 10 | Newtonian | |
| Titanium Dioxide Slurry | - | 10,000 | Thixotropic | |
| Varnish | 1.06 | 140 @ 100°F | - | |
| Turpentine | 0.86 | 2 @ 60°F | - | |
|
Paper & Textile |
Black Liquor Tar | - | 2,000 @ 300°F | - |
| Paper Coating 35% | - | 400 | - | |
| Sulfide 6% | - | 1,600 | - | |
| Black Liquor | 1.3 | 1,100 | - | |
| Black Liquor Soap | - | 7,000 @ 122°F | - | |
|
Petroleum & Petroleum Products |
Asphalt (Unblended) | 1.3 | 500 to 2,500 | - |
| Gasoline | 0.7 | 0.8 @ 60°F | Newtonian | |
| Kerosene | 0.8 | 3 @ 68°F | Newtonian | |
| Fuel Oil #6 | 0.9 | 660 @ 122°F | Newtonian | |
| Auto Lube Oil SAE 40 | 0.9 | 200 @ 100°F | Newtonian | |
| Auto Lube Oil SAE 90 | 0.9 | 320 @ 100°F | Newtonian | |
| Propane | 0.46 | 0.2 @ 100°F | Newtonian | |
| Tars | 1.2 | Wide Range | - | |
|
Pharmaceuticals |
Castor Oil | 0.96 | 350 | Newtonian |
| Cough Syrup | 1 | 190 | Newtonian | |
| Stomach" Remedy Slurries | - | 1,500 | Thixotropic | |
| Pill Pastes | - | 5,000 +/- | Thixotropic | |
|
Plastic Resins |
Butadiene | 0.94 | 0.17 @ 40°F | - |
| Polyester Resin (Typ) | 1.4 | 3,000 | Thixotropic | |
| PVA Resin (Typ) | 1.3 | 65,000 | - | |
|
Starches & Gums |
Corn Starch Sol 22°B | 1.18 | 32 | Thixotropic |
| Corn Starch Sol 25°B | 1.21 | 300 | Thixotropic | |
|
Sugar, Syrups, Molasses |
Corn Syrup 41 Be | 1.39 | 15,000 @ 60°F | Newtonian |
| Corn Syrup 45 Be | 1.45 | 12,000 @ 130°F | Newtonian | |
| Glucose | 1.42 | 10,000 @ 100°F | - | |
| Molasses A | 1.42 | 280 to 5,000 @ 100°F | - | |
| Molasses B | 1.43 to 1.48 | 1,400 to 13,000 @ 100°F | - | |
| Molasses C | 1.46 to 1.49 | 2,600 to 5,000 @ 100°F | - | |
| 60 Brix | 1.29 | 75 @ 60°F | Newtonian | |
| 68 Brix | 1.34 | 360 @ 60°F | Newtonian | |
| 76 Brix | 1.39 | 4,000 @ 60°F | Newtonian | |
| Water & Waste Treatment | Clarified Sewage Sludge | 1.1 | 2,000 Range | - |






