- Show results for
- Share
How is Conductivity Measured
Resource Description

What is Conductivity and How is It Measured
Conductivity is an inherent characteristic of the dissolved acids, bases, salts, and other dissolved solids in a liquid solution. Electrical conductivity is a good indication of the purity of the liquid. For this reason, it is commonly used in pure water measurements to determine the presence of contaminants. There are liquid solutions between the two extremes. The electrical current is transmitted by electrons in metals, but in water, it is transmitted by charged ions.
At any rate, the conductivity is measured by the amount of charge carries, how fast they are transmitted, and the power of the transmitter. The higher the ion concentration in the water solution is, the higher the conductivity is.
The conductivity boosts with the ion concentration until the liquid solution becomes too thick, thus making ions too hard to move.
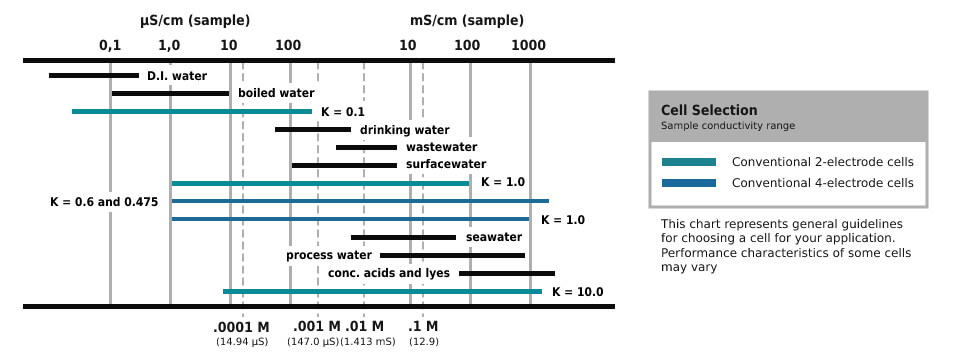
Conductance is specified as the two-way resistance and is measured in Siemens (S). The measurement results of the liquid solutions are changed to conductivity. It is done by measuring the cell constant (K) for every mounting by using a conductivity standard solution.
Conductivity = Cell Conductance X Cell Constant
The cell consists of 2 flat parallel measuring electrodes that are separated by a fixed distance. The cell constant is relevant to the physical characteristics of cell measuring and separation distance function that is split by the electrode area. The determined cell constant is a set value and is launched into the meter by which the transformation from conductance to conductivity is estimated and presented.
Conductivity is also an effective tool to measure the concentration of an acid or a base. The connection of the concentration addition of an acid or base to pure water can be charted by a well-defined curve of conductivity versus concentration. These measurements are often used to create solutions such as NaOH for clean-in-place applications.
Why is Conductivity Important
Aspects that impact water volume, for instance, heavy rains or evaporation, impact the conductivity. Drainage or flooding over soils that increase in salts and minerals may provoke a conductivity boost in addition to the water flow increase.
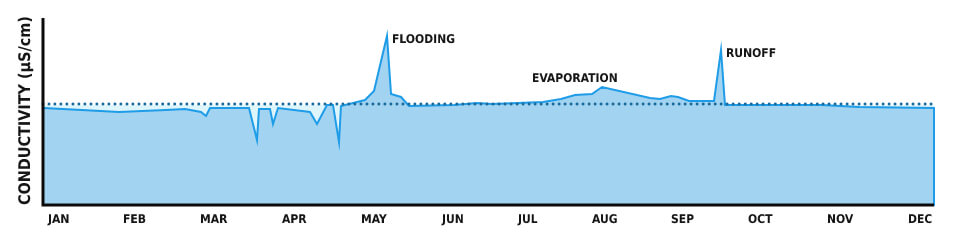
Conductivity, in a certain conductance, is the most valuable and often measured water quality criteria. Conductivity is the first indicator in water system changing, as well as the foundation for most salinity and total dissolved solids calculations.
The expanse of water maintains rather constant conductivity that can be used as a foundation for future measurement comparisons. A considerable change can be hazardous for the water quality, whether it is due to flooding, evaporation, or man-made disasters.
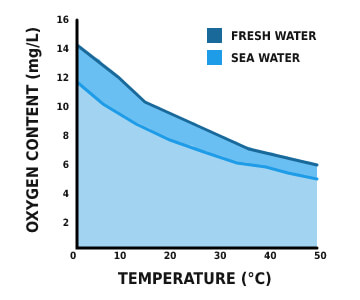
*Note: seawater can’t include as many dissolved solids as freshwater does due to high salinity.
Conductivity and salinity are strongly correlated. When conductivity is quite easy to determine, it is used the algorithms where the salinity and TDS, that are both impact the water quality, are calculated.
Salinity is significant in a certain situation as it impacts dissolved oxygen solvability. The higher the level of salinity is, the lower the concentration of dissolved oxygen. Oxygen is 20% less solvable in seawater than in freshwater at the same temperature. Average, freshwater has a higher concentration of dissolved oxygen than seawater.
How to Measure Conductivity
A conductivity meter is a tool that is used for the accurate measurements of conductivity. When the device is equipped with certain electrodes, the conductivity may be measured precisely.
Turn on the conductivity meter and check each plug to be sure that they work properly.
Take the conductivity meter’s electrode and place it into the sample you want the measure.
Press the READ button and start the measurement.
You will see the measurement results on the display. When the measurement becomes stable, you’ll get your finished results.
Tips for Proper Conductivity Measuring
The endpoint of the electrode shouldn’t be wet, not to get inaccurate measurement results.
The box or container, where you are going to put the solution for the test, should be clean and ion contamination free.
Note that the high-purity water must be measured fast after you fill it into the container or box to prevent high and wrong conductivity results due to CO2 dissolving into the water and becoming carbonate ions.







